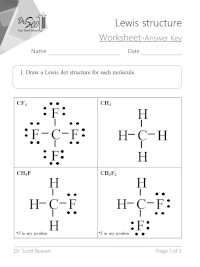
Lewis Dot Structure
Aug 15, 2020 A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as 'dots' or for bonding electrons as a line between the two atoms.
Learning Objectives
Lewis Dot Structure (Electron Dot Structure) A Lewis dot structure is a quick and easy diagram that shows the valence electrons in an element. In a Lewis structure, the nucleus of the element is represented by its symbol. The valence electrons are represented by dots placed around the symbol in pairs. Lewis Structures 1) Write the element symbol. 2) Carbon is in the 4th group, so it has 4 valence electrons. 3) Starting at the right, draw 4 electrons, or dots, counter-clockwise around the element symbol. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as 'dots' or for bonding electrons as a line between the two atoms. WRITING LEWIS DOT STRUCTURES Lewis structure or formula shows electron‐dot symbols for the atoms, the bonding pairs as lines, and the lone pairs that fill each atom's outer level (valence shell) as pairs of dots. Determine the total number of valence electrons. Add up the valence electrons of the atoms.
By the end of this section, you will be able to:
- Write Lewis symbols for neutral atoms and ions
Lewis Symbols of Monoatomic Elements
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. (It does not matter what order the positions are used.)
For example, the Lewis electron dot diagram for calcium is simply
Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table.
Figure 1. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table.
Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium:Likewise, they can be used to show the formation of anions from atoms, as shown below for chlorine and sulfur:Figure 2 demonstrates the use of Lewis symbols to show the transfer of electrons during the formation of ionic compounds.Figure 2. Cations are formed when atoms lose electrons, represented by fewer Lewis dots, whereas anions are formed by atoms gaining electrons. The total number of electrons does not change.
Example 1: Writing Lewis DoT SYmbols of Elements
What is the Lewis electron dot diagram for each element?
- aluminum
- selenium
The valence electron configuration for aluminum is 3s23p1. So it would have three dots around the symbol for aluminum, two of them paired to represent the 3s electrons (or three single dots around the atom):
The valence electron configuration for selenium is 4s24p4. In the highest-numbered shell, the n = 4 shell, there are six electrons. Its electron dot diagram is as follows:
Check Your Learning
What is the Lewis electron dot diagram for each element?
- phosphorus
- argon
Example 2: Writing Lewis DoT SYmbols of Ions
What is the Lewis electron dot diagram for each ion?
- Ca2+
- O2−
Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca2+.
Ca2+
The O2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows:
Check Your Learning
The valence electron configuration of thallium, whose symbol is Tl, is 6s25d106p1. What is the Lewis electron dot diagram for the Tl+ ion?
Key Takeaways
- Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol.
- Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom.
Exercises
1. Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol.
2. Is it necessary for the first dot around an atomic symbol to go on a particular side of the atomic symbol?
3. What column of the periodic table has Lewis electron dot diagrams with two electrons?
4. What column of the periodic table has Lewis electron dot diagrams that have six electrons in them?
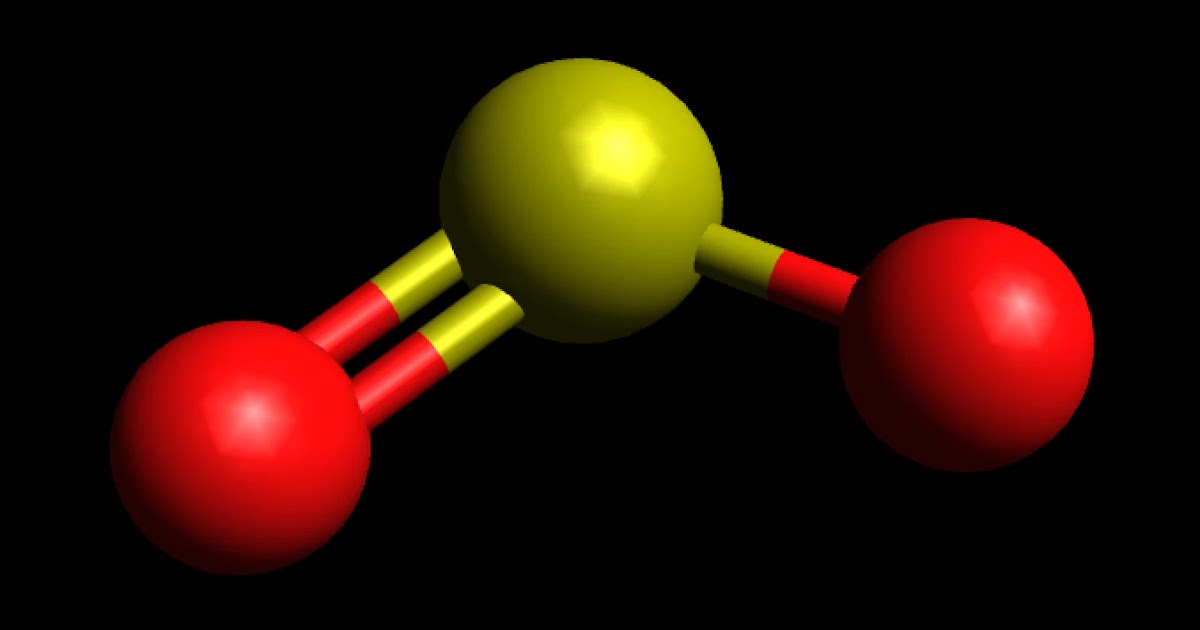
Lewis Dot Structure For Co2
5. Draw the Lewis electron dot diagram for each element.
a) strontium
b) silicon
6. Draw the Lewis electron dot diagram for each element.
a) krypton
b) sulfur
7. Draw the Lewis electron dot diagram for each element.
a) titanium
b) phosphorus
8. Draw the Lewis electron dot diagram for each element.
a) bromine
b) gallium
9. Draw the Lewis electron dot diagram for each ion.
a) Mg2+
Lewis Dot Structure For H2o
b) S2−
10. Draw the Lewis electron dot diagram for each ion.
a) In+
b) Br−
11. Draw the Lewis electron dot diagram for each ion.
a) Fe2+
b) N3−
12. Draw the Lewis electron dot diagram for each ion.
a) H+
b) H−
Show Select Answer1. The first two electrons in a valence shell are s electrons, which are paired.
3. The second column of the periodic table
5.
a)
b)
7.
a)
b)
9.
a) Mg2+
b)
11.
a) Fe2+
b)